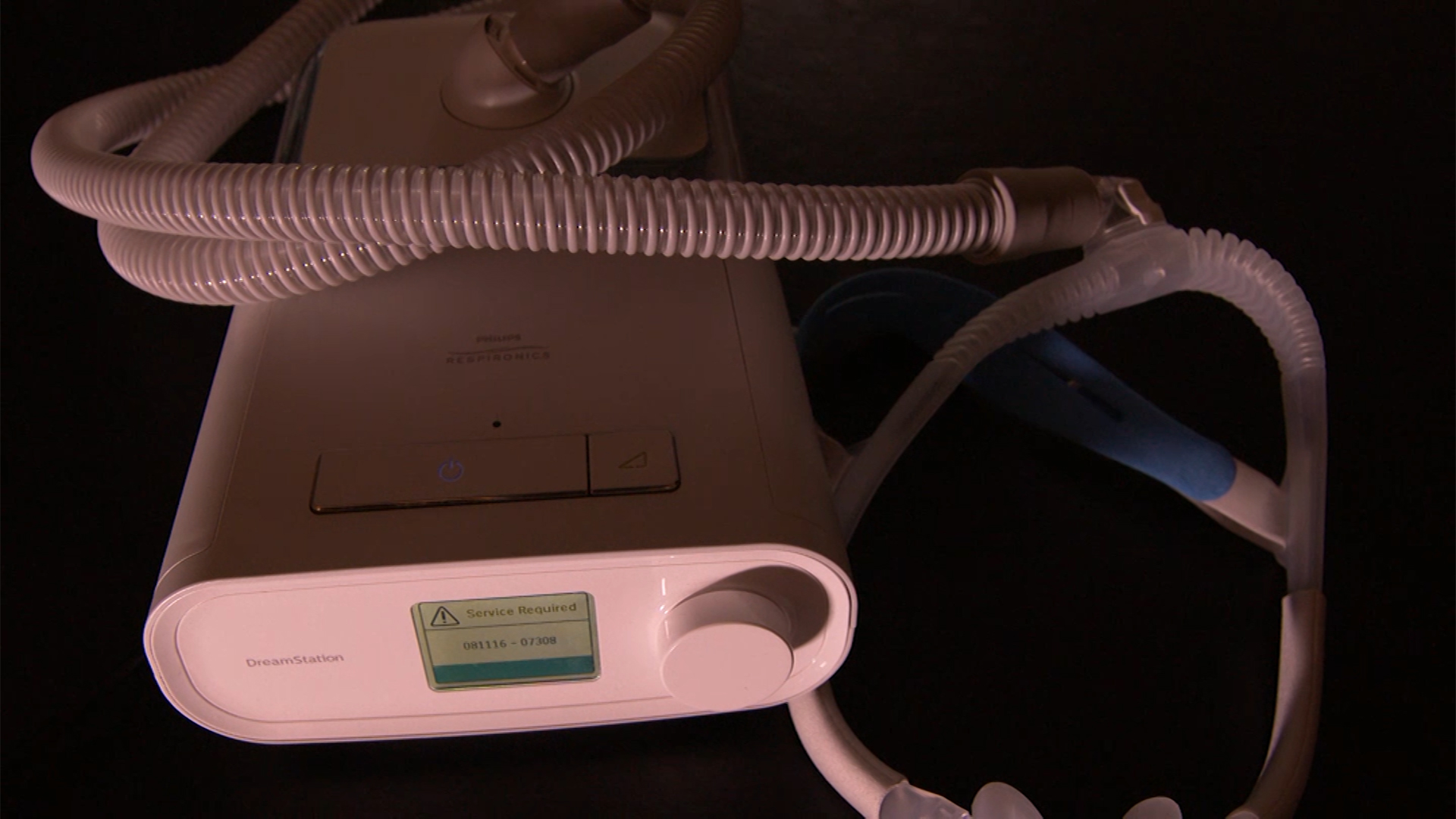
Millions of people across the country were jostled awake this year over fearful news: A critical device they were prescribed and relied on each night for sleep was suddenly labeled a hazard to their health.
A massive voluntary recall suddenly took many sleep aid devices off the market without offering a replacement.
Now, newly released documents are raising questions over whether the company at the center of that recall knew of the problems and risks with its devices for years, but did not immediately act.
For those diagnosed with sleep apnea, a condition where a person can stop breathing while sleeping, the Philips Respironics’ CPAP, BiPAP and ventilator machines were the most popular and often the go-to choice for doctors prescribing a sleep aid device.
That’s why it came as a shock when Philips Respironics announced a voluntary recall of more than 15 million devices in June. Half of those devices are in use in the U.S., the company said previously.







